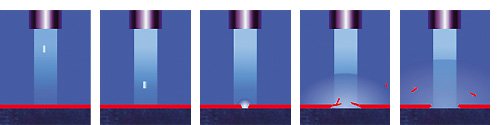
Even though the two words may seem mutually exclusive, it is exactly what makes dry ice special. As opposed to normal ice, dry ice is not made by freezing water, but out of pure carbon dioxide (CO2). The CO2 is pumped into a piston where it is subsequently compressed with a pressure of 180bar. The application of the pressure causes the carbon dioxide to change its physical state from liquid to solid as it cools down to a temperature of -78,5°C (-109.3°F). This extreme cold makes dry ice dangerous to handle without an appropriate protection due to burns caused by freezing. When the carbon dioxide reaches its solid state, it can be forms into pellets, nuggets or blocks. Because it does not consist of water, the “melting process” – sublimation – takes place without any residue, as the dry ice changes directly from its solid to gaseous state.
Dry ice is truly a versatile and well-rounded product. Given a temperature as low as -78oC, it can be used as an extremely helpful cooling agent or for dry ice blasting in industries.
Its rising popularity has also entered into private households as more and more customers rely on dry ice for cooling. From camping trips to catering, dry ice in a well-isolated container can keep your food and beverages cold up to several days.